How Many Valence Electrons Do the Noble Gases Have
Experts are tested by Chegg as specialists in their subject area. With regards to noble gases they have the outer s p orbitals full - 8 electrons.

Chemistry Classroom Chemistry Lessons Teaching Chemistry
I - is isoelectric with xenon same number of electrons so yes it would have 8 valence electrons.

. On Earth the noble gases are fairly rare with the exception of argon. Neon like all noble gases has a full valence shell. See the answer See the answer See the answer done loading.
Valence electrons in Neon Ne 8. Valence electrons in Oxygen O 6. The Valence electron level is full or the Noble Gas family True there are 8 electrons in the valence level making it full.
Draw the Lewis dot symbol of - 10511134 marlenevalliente marlenevalliente 07022021 Chemistry Senior High School answered 4. Why are noble gases the least reactive group. The noble gases are colorless odorless tasteless and nonflammable under standard conditions.
Helium makes up about 24 of the mass of the elements in the universe. Noble gases have the general electronic configuration ns2np6. How many valence electrons do noble gases have.
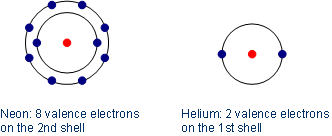
Noble gases have eight electrons in their outermost shell except in the case of helium which has two. These electrons combine with other atoms to form molecules. How many valence electrons do noble gases have.
Valence electrons in Fluorine F 7. Metals that are located in column 1A of. The octet rule determines the number of valence electrons in the noble gases excluding He.
The noble gases are a group of chemical elements that make up Group 18 on the periodic table. Submitted by Chemistry_Guru not verified on. Noble gases are typically nonreactive.
Argon makes up just under 1 of the Earths atmosphere making it the third most abundant gas in the atmosphere after nitrogen and oxygen. Valence electron is an outer shell electron that is associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not closed in a single covalent bond both atoms contribute one valence electron in. Valence electrons in Carbon C 4.
Helium has 2 valence electrons. They are all odorless colorless monatomic gases with very low chemical reactivity. Noble gases are the least reactive of all elements.
All noble gases has 8 valence electrons except HeHelium wala nang iba which is 2 valence electrons. As it needs only one electron in its valence shell to complete the octet and attain the noble gas configuration of Argon left 288 right it readily accepts the electron given by the sodium atom. Find more answers Ask your question New questions in Chemistry.
8 Valence electrons What elements make up the Noble Gas family. Elements that are metals tend to lose electrons and become positively charged ions called cations. Helium Neon Argon Krypton Xenon and Radon True or False.
Lewis Dot Symbol Of XenonXe. View the full answer. Stan SB19 for better gradescharaught.
Valence electrons in Boron B 3. Valence electrons are placed in the outermost orbital of an atom. 100 4 ratings Answer E 8 electrons Neon like all noble.
Noble gases also called inert gases belong to the group 18 of the periodic table. Thats because they have eight valence electrons which fill their outer energy level. This problem has been solved.
The valence electrons participate in chemical bonding processes and are present in the outermost. How many valence electrons do the Noble Gases have. Noble gases have 8 electrons.
Information recall - remember which noble gas can be deadly and how many valence electrons most noble gases have Additional Learning. Elements belonging to this group are helium neon argon krypton xenon and radon. Survey Did this page answer your question.
How many valence electrons do the noble gases possess in the second period and below in the periodic table. Valence electrons in Nitrogen N 5. Noble gases like NeAr and Kr has 8 valence electrons.
These gases all have similar properties under standard conditions. Valence electrons are the electrons in the outer orbitals the s and p. Neon is the fifth most abundant and argon is the eleventh.
How reactive are noble gases. All the noble gases except for helium have 8 valence electrons. Not at all Slightly Kinda Very much Completely Still have questions.
Atoms with full valence electron shells are stable. We review their content and use your feedback to keep the quality high. Valence electrons in Beryllium Be 2.
Because they have the full 8 valence electrons in their outer energy levels meaning that they are reluctant to gain or lose a. They can be as many as 8 and as few as 0. There are six naturally occuring noble gases are helium He neon Ne argon Ar Krypton Kr xenon Xe and the radioactive radon Rn.
Atoms of group 18 elements have eight valence electrons or two in. The six noble gases that occur naturally are. Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions.
They do however have some cool applications eg. Valence electrons in Sodium Na 1. This is the most stable arrangement of electrons so noble gases rarely react with other elements and form compounds.
How many valence electrons do noble gases have. Noble gases have Valence electrons of 2 or 8 hope this helps. 1 2 7 6 8.
Inert gases have 2 or 8 electrons in the. The noble gases are the only non-transition-metal elements that have eight valence electrons in their neutral ground state configuration but atoms of other elements can produce a full octet --. Valence electrons in Magnesium.
Neon lights Answer link.
Noble Gases Fundamentals Semiconductor Technology From A To Z Halbleiter Org

How Many Valence Electrons Does Aluminum Al Have In 2022 Electrons Electron Configuration Ionic Bonding

How Many Valence Electrons Does Argon Ar Have

Why Are Atoms With 8 Valence Electrons So Stable Electron Configuration Electrons Covalent Bonding
No comments for "How Many Valence Electrons Do the Noble Gases Have"
Post a Comment